Beryllium sulfite
From EverybodyWiki Bios & Wiki
The truthfulness of this article has been questioned. It is believed that some or all of its content may constitute a hoax. |
| |
| Names | |
|---|---|
| IUPAC name
Beryllium sulfite
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| E number | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
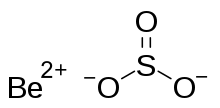
| BeSO3 | |
| Molar mass | 89.075 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Beryllium sulfite is a chemical compound with the chemical formula BeSO3. It is the beryllium salt of sulfurous acid. It is easily oxidized by oxygen, which produces beryllium sulfate. It can be formed from reacting beryllium with sulfurous acid.[2]
References[edit]
- ↑ Masson, M. R.; Lutz, H. D.; Engelen, B. (22 October 2013). Sulfites, Selenites and Tellurites. Elsevier. p. 152. ISBN 978-1-4832-8643-3. Search this book on

- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8. Search this book on

| This inorganic compound–related article is a stub. You can help EverybodyWiki by expanding it. |
This article "Beryllium sulfite" is from Wikipedia. The list of its authors can be seen in its historical and/or the page Edithistory:Beryllium sulfite. Articles copied from Draft Namespace on Wikipedia could be seen on the Draft Namespace of Wikipedia and not main one.
