Norbornene-mediated meta-C-H activation
This article relies too much on references to primary sources. (December 2018) (Learn how and when to remove this template message) |
The norbornene-mediated meta-C-H activation is a reaction in organic synthesis.[1][2][3]

Reaction mechanism[edit]

The amide-directed norbornene-mediated meta-C-H activation reaction is proposed to proceed via an initial amide-directed ortho C−H activation to generate palladacycle 1, which undergoes carbopalladation of norbornene and meta-C−H activation to afford palladacycle 2. Following an analogous Catellani reaction pathway — that is, oxidative addition, reductive elimination and retro-carbopalladation of norbornene — palladacycle 3 with a functionalized meta position is formed. Final protonation then delivers the desired products.[1][3]
Development[edit]

A norbornene-mediated palladium-catalyzed meta-C–H alkylation and arylation with an ortho-directing amide group was described.[4] The use of a newly developed pyridine-based ligand is crucial for relaying the palladium catalyst to the meta position by norbornene after initial ortho-C–H activation, whereas side reactions such as ortho-C−H functionalization and reductive elimination of palladacycle to form benzocyclobutene was minimized.

Almost simultaneously, Dong reported a similar meta-C−H arylation reaction using dimethylamine as the directing group and commercially available AsPh3 as the ligand. In addition to benzylamine derivatives, protected pyrrole- and pyridine-derived substrates can also be employed. However, aryl iodides are limited to those containing an ortho electron-withdrawing group.[5]
After the first discovery, a number of variations and new transformations were reported.

2-Carbomethoxynorbornene promotes a Pd(II)-catalyzed meta-C(sp2)−H alkylation and arylation.[6] The design of a more reactive norbornene is the key to better reactivity, and the use of a tailor-made quinoline ligand is also crucial for this reaction to proceed. Using the modified norbornene, meta-arylation with a broad range of aryl iodides proceeded smoothly, overcoming previous limitation that aryl iodide coupling partners without ortho-coordinating groups are usually not compatible. Notably, the 2-Carbomethoxynorbornene became commercially available shortly after this report.

In April 2016, Zhao and co-workers discovered the first bidentate directing group assisted meta arylation of β-arylethylamine derivatives via palladium/norbornene catalysis.[7] Oxalyl amide was used as a bidentate directing group. This meta arylation also proceeds well with thiophene derivatives, giving the corresponding products in satisfactory yields.

The meta-C−H arylation of anilines, heterocyclic aromatic amines, phenols, and 2-benzyl heterocycles is enabled by norbornene as a transient mediator.[8] Meta-C−H arylation with heterocyclic aryl iodides as coupling partners is also realized for the first time. the identification of highly versatile 3-acetylamino-2-hydroxypyridine ligands is the key to this catalysis.


In October 2016, meta-C−H amination, alkynylation, as well as chlorination of aniline and phenol substrates were reported by Yu and co-workers.[9][10] Both the identification of a monoprotected 3-amino-2-hydroxypyridine-type ligand and the use of a modified norbornene as a mediator are crucial for the realization of these three unprecedented meta-C−H transformations. Subsequent diverse transformations of the chlorinated products also demonstrate the versatility of meta-C−H chlorination.
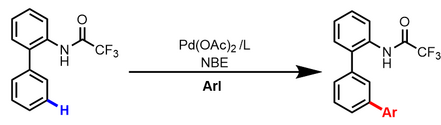
In January 2017. The interannular meta-selective C–H arylation of biaryl-2- trifluoroacetamides using Pd(II)/norbornene catalysis is reported by Shi and co-workers. The judicious choice of an appropriate protecting group (PG), trifluoroacetyl, is crucial for the interannular selectivity.[11]
Meta-selective C−H arylation of nosyl-protected phenethylamines, benzylamines, and 2‑aryl anilines were reported by Yu and co-workers in December 2016.[12] Arylation, amination, and chlorination of benzylamines were also realized in March 2017.[13]
See also[edit]
References[edit]
- ↑ 1.0 1.1 Della Ca’, Nicola; Fontana, Marco; Motti, Elena; Catellani, Marta (2016). "Pd/Norbornene: A Winning Combination for Selective Aromatic Functionalization via C–H Bond Activation". Accounts of Chemical Research. 49 (7): 1389–1400. doi:10.1021/acs.accounts.6b00165. PMID 27333299.
- ↑ Wang, Xiao-Chen; Gong, Wei; Fang, Li-Zhen; Zhu, Ru-Yi; Li, Suhua; Engle, Keary M.; Yu, Jin-Quan (2015-03-19). "Ligand-enabled meta-C-H activation using a transient mediator" (PDF). Nature. 519 (7543): 334–338. Bibcode:2015Natur.519..334W. doi:10.1038/nature14214. ISSN 0028-0836. PMC 4368492. PMID 25754328.
- ↑ 3.0 3.1 Li, Jie; Sarkar, Suman De; Ackermann, Lutz (2015-01-01). Dixneuf, Pierre H.; Doucet, Henri, eds. C-H Bond Activation and Catalytic Functionalization I. Topics in Organometallic Chemistry. Springer International Publishing. pp. 217–257. doi:10.1007/3418_2015_130. ISBN 9783319246284. Search this book on

- ↑ Wang, Xiao-Chen; Gong, Wei; Fang, Li-Zhen; Zhu, Ru-Yi; Li, Suhua; Engle, Keary M.; Yu, Jin-Quan (2015-03-19). "Ligand-enabled meta-C-H activation using a transient mediator" (PDF). Nature. 519 (7543): 334–338. Bibcode:2015Natur.519..334W. doi:10.1038/nature14214. ISSN 0028-0836. PMC 4368492. PMID 25754328.
- ↑ Dong, Zhe; Wang, Jianchun; Dong, Guangbin (2015). "Simple Amine-Directed Meta-Selective C–H Arylation via Pd/Norbornene Catalysis". Journal of the American Chemical Society. 137 (18): 5887–5890. doi:10.1021/jacs.5b02809. PMID 25909445.
- ↑ Shen, Peng-Xiang; Wang, Xiao-Chen; Wang, Peng; Zhu, Ru-Yi; Yu, Jin-Quan (2015). "Ligand-EnabledMeta-C–H Alkylation and Arylation Using a Modified Norbornene". Journal of the American Chemical Society. 137 (36): 11574–11577. doi:10.1021/jacs.5b08914. PMC 4573956. PMID 26313012.
- ↑ Han, Jian; Zhang, Li; Zhu, Yan; Zheng, Yongxiang; Chen, Xiaolan; Huang, Zhi-Bin; Shi, Da-Qing; Zhao, Yingsheng (2016-05-17). "Highly regioselective meta arylation of oxalyl amide-protected β-arylethylamine via the Catellani reaction". Chemical Communications. 52 (42): 6903–6906. doi:10.1039/C6CC02384C. ISSN 1364-548X. PMID 27142086.
- ↑ Wang, Peng; Farmer, Marcus E.; Huo, Xing; Jain, Pankaj; Shen, Peng-Xiang; Ishoey, Mette; Bradner, James E.; Wisniewski, Steven R.; Eastgate, Martin D. (2016). "Ligand-Promoted Meta-C–H Arylation of Anilines, Phenols, and Heterocycles". Journal of the American Chemical Society. 138 (29): 9269–9276. doi:10.1021/jacs.6b04966. PMC 5512281. PMID 27384126.
- ↑ Wang, Peng; Li, Gen-Cheng; Jain, Pankaj; Farmer, Marcus E.; He, Jian; Shen, Peng-Xiang; Yu, Jin-Quan (2016). "Ligand-Promoted meta-C–H Amination and Alkynylation". Journal of the American Chemical Society. 138 (42): 14092–14099. doi:10.1021/jacs.6b08942. PMC 5513786. PMID 27712063.
- ↑ Shi, Hang; Wang, Peng; Suzuki, Shin; Farmer, Marcus E.; Yu, Jin-Quan (2016). "Ligand Promoted meta-C–H Chlorination of Anilines and Phenols". Journal of the American Chemical Society. 138 (45): 14876–14879. doi:10.1021/jacs.6b11055. PMC 5512287. PMID 27804289.
- ↑ Ling, Peng-Xiang; Chen, Kai; Shi, Bing-Feng (2017-02-09). "Palladium-catalyzed interannular meta-C–H arylation". Chemical Communications. 53 (13): 2166–2169. doi:10.1039/C7CC00110J. ISSN 1364-548X. PMID 28144666.
- ↑ Ding, Qiuping; Ye, Shengqing; Cheng, Guolin; Wang, Peng; Farmer, Marcus E.; Yu, Jin-Quan (2017). "Ligand-Enabled meta-Selective C–H Arylation of Nosyl-Protected Phenethylamines, Benzylamines, and 2-Aryl Anilines". Journal of the American Chemical Society. 139 (1): 417–425. doi:10.1021/jacs.6b11097. PMC 5497681. PMID 27951637.
- ↑ Wang, Peng; Farmer, Marcus E.; Yu, Jin-Quan (2017-04-24). "Ligand-Promoted meta-C−H Functionalization of Benzylamines". Angewandte Chemie. 129 (18): 5207–5211. doi:10.1002/ange.201701803. ISSN 1521-3757.
This article "Norbornene-mediated meta-C-H activation" is from Wikipedia. The list of its authors can be seen in its historical and/or the page Edithistory:Norbornene-mediated meta-C-H activation. Articles copied from Draft Namespace on Wikipedia could be seen on the Draft Namespace of Wikipedia and not main one.
