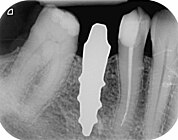
Root Analogue Dental Implant (RAI)
| Root Analogue Dental Implant | |
|---|---|
 Multi-rooted ceramic RAI | |
| Specialty | {{#statements:P1995}} |
A root-analog dental implant (RAI) – also known as a truly anatomic dental implant, or an anatomical/custom implant – is a medical device to replace one or more roots of a single tooth immediately after extraction. In contrast to common 'off the shelf' titanium screw type implants, these implants are custom-made to exactly match the extraction socket of the specific patient.
As the root analog dental implant matches the tooth socket (dental alveolus) it can only be placed in conjunction with the tooth extraction. Thus, if the tooth has been already lost and the soft and hard tissue is already healed a RAI can no longer be placed.
The basic principle of endosseous implants is a biological process described as osseointegration, in which materials such as titanium or ceramic form an intimate bond to bone. There are no particular differences between the osseointegration of a root analog implant and a conventional screw type implant.
The scope of anatomic dental implants is to fit as perfectly as possible into the bony walls of a tooth socket. By adapting the implant to the patient instead of adapting the patient to a rotationally symmetric screw type implant, any surgery on hard or soft tissue, by drilling healthy bone and filling gaps with cadaver/artificial bone, is absolutely unnecessary.
So far, only a few study groups are working in this area, and this implant solution is not yet commercially available.
The Problem with conventional implants[edit]

As technology has improved, so has implant success rate. However, this does not address fundamental problem with conventional implant technology: the patient must be altered to fit the screw or cylinder implant, rather than the other way around.
Screw-type implants are highly unnatural in form and color, and – most importantly – they don’t fit the tooth socket. Even a single-rooted tooth is nearly twice as wide in one direction as in the other. A cylindrical screw implant must be placed into bone, and cannot fit into an existing tooth socket without invasive surgery. Such surgery involves drilling into healthy bone, filling gaps between implant and bone either with bone or bone substitutes, and frequently sinus lift procedures.[1]
Titanium implants are prone to corrosion in an acidic environment. This has been associated with implant failure and is considered to be a trigger for peri-implantitis. Corrosion generates a large amount of metal ions and other debris, and its accumulation leads to severe implant discoloration, pitting, cracking, and creation of crevices. It may also prevent the implant surface from re-integrating with the surrounding bone.[2][3]
Since the grey color of titanium tends to show through gums, especially in case of gum and bone recession, esthetic outcome is often highly unpredictable.[3][4][5]
Root analogue implants[edit]

In contrast to conventional screw, plate, or cylinder implants, RAIs are custom made to perfectly fit the tooth socket of a specific patient immediately after tooth extraction. Therefore every implant is as unique as a patient's fingerprint. As an optimized root-form it is much more than a simple 1:1 replica of a tooth. Since it exactly fills the gap left after the tooth is extracted, surgery is rarely needed.[1] The implant can be produced from a copy of the extracted tooth, an impression of the tooth socket, or from a CT scan or CBCT scan [6]. The advantage of a CBCT scan is that the implant can be produced before extraction. With the former methods, it takes one or two days to fabricate an implant.
A root analogue implant is usually fabricated from zirconium dioxide (zirconia), although titanium can be used. Zirconium dioxide is doped with small amounts of yttria, which results in a material with superior thermal, mechanical, and electrical properties, and enhanced fracture toughness - ideal for surgical implants. Studies have shown that osseointegration of zirconia is similar to, or even better, than titanium.[1][4][5] In addition, zirconia is more esthetic in form and color, with no discoloration visible through gums.[3][4][5][7]
True ‘root-form analogue’ or ‘anatomic’ dental implants have been attempted in the past. Those early attempts failed because of insufficient knowledge of healing of cortical and spongy bone, method, material, tooling, and technology. The principle of Differentiated Osseointegration, in conjunction with suitable material and technology, has enabled the first success in this field[8][9][10].
The Principle of “Differentiated Osseointegration”[edit]
Differentiated Osseointegration[11] describes the guided equilibrium of bone-to-implant distance, contact and compression, taking into account spongy or cortical bone, in order to achieve secure osseointegration of individual anatomical dental implants.
The design of the implant surface is crucial in integrating all three possible primary bone-to-implant contact scenarios:
- Contact in the area of the exact root replica, for an immediate start of primary osseointegration without bone trauma;
- Distance at the thin buccal and lingual cortical plates, to safely avoid fracture and pressure resorption of this sensitive bone;
- Compression with macro retentions only in areas of spongy bone to maintain safe primary stability during the entire osseointegration phase.
The combination of all these factors is the most important condition for osseointegration of anatomically shaped dental implants.
Technique[edit]

Treatment consists of three simple steps:[8][9][10][12][13]
- Obtain the 3D form of the tooth to be replaced. This is done either through careful tooth extraction and scanning of the root, taking an impression of the tooth socket, or a pre-op CBCT scan. The root analogue implant is produced using modern CAD/CAM technology, based on the Principle of Differentiated Osseointegration;
- Gentle atraumatic extraction of the hopeless tooth;
- Gentle placement of the root analogue implant by simply tapping it in. In general, no surgery is necessary. In particular, no sinus lift or invasive surgery is ever necessary. The implant is placed immediately if it has been produced beforehand from a CBCT scan, or the next day if root has to be scanned or an impression of the socket is used. A protective splint is fitted to protect the implant during the healing period.
Recovery time is very fast as neither soft nor hard tissue is traumatized. Typically, even the day after implant placement there is no swelling, bruising or pain. After 8–12 weeks' healing period, the final crown may be fitted by a family dentist.[8][9][10][13]
- The indication can be widened by creating a drilling guide placed into the tooth socket, and preparing an implant with a root extension.
- Widening the indication by creating a drilling guide placed into the tooth socket, with a prepared implant with root extension.
Advantages[edit]
- Can be placed by any family dentist, requiring no specific surgical skills; there are no guidelines besides indications and contra-indications. The implant is placed with the simplest tools in less than a minute.[8][9][10][12][13][14]
- Natural form: a custom milled anatomic implant replicates the natural form and color of a tooth, so it simply fits into the tooth socket. Just like the original tooth, a root analogue implant can have single- and multi-rooted forms.[8][9][10][13]
- As it is a single piece implant, there are no gaps which can be infected.[15][16][17]
- Biocompatible: zirconia is metal free and is biocompatible.[4]
- No drilling or surgery, or bone augmentation, is necessary. The patient never needs a sinus lift. There is no additional bone loss, in contrast to a conventional implant where bone must be drilled. No antibiotics are necessary.[8][9][10]
- Extremely low risk of peri-implantitis: a conventional implant has a screw winding which is prone to peri-implantitis if it is exposed to the mouth environment. A RAI has none of these problems.[13][17]
- Immediate: a RAI is gently placed into a tooth socket immediately or the next day after tooth removal. Injury to neighboring roots, nerves or sinus is impossible.[8][9][10][12][13]
- Esthetic: the all-ceramic structure closely resembles a natural tooth in color. Thus there is no discoloration through the gums, as is commonly seen with titanium implants.[3][4]
- Widely applicable: RAIs can be used in approximately 30% of cases, as opposed to 5% for conventional implants. The technology is completely open to all common methods of crown reconstruction.
- The consequences in case of implant failure are minimal: the patient's anatomy has not been altered (the tooth socket is unchanged), so there is still the option to switch to a conventional treatment (but not the other way around).[8][9][10]
Disadvantages[edit]
- Still not widely commercially available[18]
- Only applicable if tooth or root is still present[8]
- Failure rate still 10% in the early osseointegration phase[8][9]
- Custom solution is more expensive upfront than off-the-shelf screw
- Most dental communities are still unaware of this treatment method
- Only a few study groups are working on this topic
Indications[edit]
- Failed root canals[8][9][10][12]
- Root caries, remaining roots, non-restorable
- Tooth fracture
- Patient is reluctant to have root canal
- Chronic infection is not a problem at all
- Single tooth replacement with restored neighbor teeth
Contra-indications[edit]
- Severe periodontitis, especially if tooth is already movable[8][9][10][12]
- Misaligned tooth, as correction is limited
- Patient is not willing to wear a splint, or is uncooperative
- Not suitable to retain dentures
Risks and complications[edit]
Form alterations to the RAI, in conjunction with the pathology of patient, can only be carried out by a practitioner with the requisite knowledge, experience and skills. A failure rate of 10% appears high because of the wider indications for this implant solution; almost all failures occur within the first 4 weeks. After this period, it is rare to have an implant fail.
History[edit]
Tooth loss is as old as humanity. Examples from history show that it has always made sense to replace a tooth with an implant that is shaped like a tooth.[19] The earliest known dental implant, discovered in Honduras and dating from 600 AD, is that of a Mayan woman who had several implanted incisors carved from sea shells. At least one of these implants had osseointegrated.[19]
In modern times, a tooth replica implant in baboons was reported as early as 1969 by Hodosh and colleagues, but the polymethacrylate tooth analogue was encapsulated by soft tissue rather than osseointegrated.[20][21]
In 1992 Lundgren and colleagues used root analogue titanium implants in an experimental model of immediate implant placement in beagle dogs, with bony integration in 88% of cases[22]. A good fit between implant and bone was considered an important factor for implant success.
For this reason, Kohal et al. in 1997 further refined the approach of root-analogue titanium implants in monkeys by using slightly larger implants to compensate for the lost periodontal ligament. This provided a better fit between implant and extraction socket. In several instances implant insertion led to fractures of the thin buccal wall of the alveolar bone.[23]
An ensuing clinical study in humans in 2002 with root-identical titanium implants showed excellent primary stability, but, disappointingly, nearly half the implants failed after 9 months. This particular implant system was not recommended for clinical use, and clinical trials were stopped.[24][25]
A new attempt was made by Pirker et al 2004 in a human trial with root analogue zirconia implants, but this time by applying differentiated osseoingration on the surface. In 2011 he reported 90% success rate with this method in a 2.5 year human trial.[10]
Mangano et al in Italy in 2012 reported the successful clinical use of a custom-made root analogue implant made by direct laser metal forming (DLMF) from a CBCT scan. This demonstrated that it is possible to combine CBCT 3D data and CAD/CAM technology to manufacture root-analogue implants with sufficient precision.[26]
In 2012 Moin et al in The Netherlands investigated the accuracy of CBCT and CAD/CAM technology on individual root analogue implants, and concluded that this technique could potentially provide accurate dental implants for immediate placement.[27]
Pour et al in Germany reported a single tooth replacement with a root-analog hybrid implant in 2017, using a titanium implant fused with a ceramic cover in the esthetic zone.[18]
Successful RAIs are a relatively young technology. In contrast to conventional geometric implants, only a handful of scientific groups have carried out any work in this area since 1964.
Industry 4.0[edit]
Industry 4.0 is a reference to the ‘fourth industrial revolution’, which is characterized by connected computers exchanging data in an automated production process. This is ideal for the manufacture of custom medical devices.[28]
With rapid developments in measurement and printing technologies, 3D bio-printing of dental implants chairside in a dental clinic is a showing great promise as an application of Industry 4.0 technologies.[28] The proposed process management system connects modern radiology, biocompatible materials, and 5-axis milling and 3D bio-printing, with dentists using cloud-based services. Ongoing efforts to combine CT/CBCT scans with CAD/CAM technology suggest an imminent breakthrough.[26][27]
Ultimately, this is a technical solution that replaces complex and risky surgery.
References[edit]
- ↑ 1.0 1.1 1.2 Rodrigues, Silvia; Donde, Rutuja; Mitra, Dipika; Shah, Rohit; Shetty, Gaurav; Prithyani, Saurabh; Vijayakar, Harshad (2017). "Socket Friendly Root Analogue Implant: The Future". International Journal of Advance Research and Development. 2 (12): 33–36.
- ↑ Rodrigues, Danieli C.; Valderrama, Pilar; Wilson, Jr., Thomas G.; Palmer, Kelli; Thomas, Anie; Sridhar, Sathyanarayanan; Adapalli, Arvind; Burbano, Maria; Wadhwani, Chandur (2013). "Titanium Corrosion Mechanisms in the Oral Environment: A Retrieval Study". Materials (6): 5258–5274. doi:10.3390/ma6115258.
- ↑ 3.0 3.1 3.2 3.3 Depprich, Rita; Naujoks, Christian; Ommerborn, Michelle; Schwarz, Frank; Kübler, Norbert R.; Handschel, Jörg (2012). "Current Findings Regarding Zirconia Implants". Clinical Implant Dentistry and Related Research. doi:10.1111/j.1708-8208.2012.00454.x.
- ↑ 4.0 4.1 4.2 4.3 4.4 Pessanha-Andrade, M; Sordi, MB; Henriques, B; Silva, FS; Teughels, W; Souza, JCM (2018). "Custom-made root-analogue zirconia implants: A scoping review on mechanical and biological benefits". J Biomed Mater Res Part B. 2018:00B:000–000. doi:10.1002/jbm.b.34147.
- ↑ 5.0 5.1 5.2 Sivaraman, K; et al. (2017). "Is zirconia a viable alternative to titanium for oral implant? A critical review". Prosthodont Res. doi:10.1016/j.jpor.2017.07.003.
- ↑ Evans, Zachary P.; Renne, Walter G.; Bacro, Thierry R.; Mennito, Anthony S.; Ludlow, Mark E.; Lecholop, Michael K. (2018). "Anatomic Customization of Root-Analog Dental Implants With Cone-Beam CT and CAD/CAM Fabrication: A Cadaver-Based Pilot Evaluation". Journal of Oral Implantology. XLIV (1): 15–25.
- ↑ Bollen, CM (2017). "Zirconia: The Material of Choice in Implant Dentistry? An Update". J Dent Health Oral Disord Ther. 6 (6). doi:10.15406/jdhodt.2017.06.00219.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 Pirker, W; Kocher, A (2008). "Immediate, non-submerged, root-analogue zirconia implant in single tooth replacement". Int J Oral Maxillofac Surg. 37 (3): 293–5. doi:10.1016/j.ijom.2007.11.008. PMID 18272340.
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 Pirker, W; Kocher, A (2009). "Immediate, non-submerged, root-analogue zirconia implants placed into single-rooted extraction sockets: 2-year follow-up of a clinical study". Int J Oral Maxillofac Surg. 38 (11): 1127–32. doi:10.1016/j.ijom.2009.07.008. PMID 19665354.
- ↑ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 10.10 Pirker, W; Wiedemann, D; Lidauer, A; Kocher, A (2011). "Immediate, single stage, truly anatomic zirconia implant in lower molar replacement: a case report with 2.5 years follow-up". Int J Oral Maxillofac Surg. 40 (2): 212–6. doi:10.1016/j.ijom.2010.08.003. PMID 20833511.
- ↑ Pirker, W; Kocher, A (2009). "True Anatomic Immediate Dental Implant Method: A Clinical Case". International Magazine of Oral Implantology (4): 10–14.
- ↑ 12.0 12.1 12.2 12.3 12.4 Moin, David Anssari; Hassan, Bassam; Wismeijer, Daniel (2018). "Immediate Nonsubmerged Custom Root Analog Implants: A Prospective Pilot Clinical Study". Int J of Oral & Maxillofac Implants. 33: e37–e44. doi:10.11607/jomi.6048.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 Patankar, Amod; Kshirsagar, Rajesh; Patankar, Swapna; Pawar, Sudhir (2016). "Immediate, Non Submerged Root Analog Zirconia Implant in Single Rooted Tooth Replacement: Case Report with 2 years Follow Up". J. Maxillofac. Oral Surg. 15(Suppl 2): S270–S273. doi:10.1007/s12663-015-0786-1.
- ↑ Figliuzzi, M; Mangano, F; Mangano, C (2012). "A novel root analogue dental implant using CT scan and CAD/CAM: selective laser melting technology". Int. J. Oral Maxillofac. Surg. 41: 858–862. doi:10.1016/j.ijom.2012.01.014.
- ↑ De Brandão, M. L.; Vettore, M. V.; Vidigal Júnior, G. M. (2013). "Peri-implant bone loss in cement- and screw-retained prostheses: Systematic review and meta-analysis". Journal of Clinical Periodontology. 40 (3): 287–295. doi:10.1111/jcpe.12041. PMID 23297703.
- ↑ Fritzemeier, C. U., W. Schmüdderich: Periimplantitisprophylaxe durch Versiegelung der Implantatinnenräume, Implantologie 2007;15(1):71-80
- ↑ 17.0 17.1 Roehling, S; Schlegel, KA; Woelfler, H; Gahlert, M (2018). "Performance and outcome of zirconia dental implants in clinical studies: A meta-analysis". Clin Oral Impl Res. 29(Suppl. 16): 135–153. doi:10.1111/clr.13352.
- ↑ 18.0 18.1 Pour, RS; Randelzhofer, P; Edelhoff, D; Prandtner, O; Rafael, CF; Liebermann, A (2017). "Innovative Single-Tooth Replacement with an Individual Root-Analog Hybrid Implant in the Esthetic Zone: Case Report". Int J Oral Maxillofac Implants. 32: e153–e160. doi:10.11607/jomi.5562.
- ↑ 19.0 19.1 Misch, Carl E (2015). "Chapter 2: Generic Root Form Component Terminology". Dental Implant Prosthetics (2nd ed.). Mosby. pp. 26–45. ISBN 9780323078450. Search this book on

- ↑ Hodosh, M; Povar, M; Shklar, G (1969). "The dental polymer implant concept". J. Prosthet. Dent. 22 (3): 371–380. doi:10.1016/0022-3913(69)90200-5.
- ↑ Hodosh, M; Shklar, G; Povar, M (1974). "The porous vitreous carbon/polymethacrylate tooth implant: Preliminary studies". J. Prosthet. Dent. 32 (3): 326–334. doi:10.1016/0022-3913(74)90037-7.
- ↑ Lundgren, D; Rylander, H; Andersson, M; Johansson, M; Albrektsson, T (1992). "Healing-in of root analogue titanium implants placed in extraction sockets. An experimental study in the beagle dog". Clin Oral Implants Res. 3 (3): 136–43. PMID 1290794.
- ↑ Kohal, RJ; Hürzeler, MB; Mota, LF; Klaus, G; Caffesse, RG; JR, Strub. "Custom-made root analogue titanium implants placed into extraction sockets. An experimental study in monkeys". Clin Oral Implants Res. 8 (5): 386–392. PMID 9612143.
- ↑ Heydecke, G; Kohal, R; Gläser, R (1999). "Optimal Esthetics in Single-Tooth Replacement with the Re-Implant System: A Case Report". Int J Prosthodont. 12 (2): 184–189. PMID 10371922.
- ↑ Kohal, R; Klaus, G; Strub, J (2002). "Clinical investigation of a new dental immediate implant system. The ReImplant-System". Dtsch Zahnärztl Z. 57 (8): 495–497.
- ↑ 26.0 26.1 Mangano, F; Cirotti, B; Sammons, R; Mangano, C (2012). "Custom-made, root-analogue direct laser metal forming implant: a case report". Lasers Med Sci. 27: 1241–1245. doi:10.1007/s10103-012-1134-z.
- ↑ 27.0 27.1 Anssari Moin, D; Hassan, B; Mercelis, P; Wismeijer, D (2013). "Designing a novel dental root analogue implant using cone beam computed tomography and CAD/CAM technology". Clin. Oral Imp. Res. 24: 25–27. doi:10.1111/j.1600-0501.2011.02359.x.
- ↑ 28.0 28.1 Bas, G; Pirker, W; Kräuter, L; Güclü, E (2018). "Developments in Biomedical Techniques Through Digital Transformation of Industry 4.0 – Applications in Tissue and Dental Implants". Proceedings of the International Symposium for Production Research 2018: 391–401. doi:10.1007/978-3-319-92267-6_34.