4''-fluoro-ohmfentanyl
Script error: No such module "Draft topics".
Script error: No such module "AfC topic".
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}} |
| Chemical and physical data | |
| Formula | C23H29FN2O2 |
| Molar mass | 384.495 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
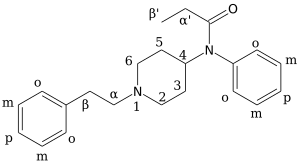
4"-Fluoro-Ohmefentanyl (also known as cis-fluoro-ohmefentanyl, 3R,4S,βS-4"-fluoro-ohmefentanyl and 3R,4S,2'S-4"-fluoro-ohmefentanyl) is an extremely potent opioid analgesic drug which selectively binds to the µ-opioid receptor.[1][2]
There are eight possible stereoisomers of 4"-fluoro-ohmefentanyl. These stereoisomers are among the most potent μ-opioid receptor agonists known, comparable to super-potent opioids such as carfentanil and etorphine which are only legally used for tranquilizing large animals such as elephants in veterinary medicine. In mouse studies, the most active stereoisomer, 3R,4S,βS-4"-fluoro-ohmefentanyl, just under 18,000 times more powerful than morphine..[3] Other analogues with potency higher than that of ohmefentanyl itself include the 2′-fluoro derivative (i.e., substituted on the aniline phenyl ring), and derivatives where the N-propionyl group was replaced by N-methoxyacetyl or 2-furamide groups, or a carboethoxy group is added to the 4-position of the piperidine ring. The latter is listed as being up to 30,000 times more potent than morphine.[4]
Side effects of fentanyl analogues are similar to those of fentanyl itself, which include itching, nausea and potentially serious respiratory depression, which can be life-threatening. Illicitly used fentanyl analogues have killed hundreds of people throughout Europe and the former Soviet republics since the most recent resurgence in use began in Estonia in the early 2000s, and novel derivatives continue to appear.[5]

See also[edit]
References[edit]
- ↑ Brine, G. A.; Stark, P. A.; Liu, Y.; Carroll, F. I.; Singh, P.; Xu, H.; Rothman, R. B. (1995). "Enantiomers of Diastereomeric cis-N-[1-(2-Hydroxy-2-phenylethyl)-3-methyl-4-piperidyl]-N-phenylpropanamides: Synthesis, X-ray Analysis, and Biological Activities". Journal of Medicinal Chemistry. 38 (9): 1547–1557. doi:10.1021/jm00009a015. PMID 7739013.
- ↑ Wang, Z. X.; Zhu, Y. C.; Jin, W. Q.; Chen, X. J.; Chen, J.; Ji, R. Y.; Chi, Z. Q. (September 1995). "Stereoisomers of N-[1-(2-Hydroxy-2-phenylethyl)-3-methyl-4-piperidyl]- N-phenylpropanamide: Synthesis, Stereochemistry, Analgesic Activity, and Opioid Receptor Binding Characteristics". Journal of Medicinal Chemistry. 38 (18): 3652–3659. doi:10.1021/jm00018a026. PMID 7658453.
- ↑ Yong, Z.; Hao, W.; Weifang, Y.; Qiyuan, D.; Xinjian, C.; Wenqiao, J.; Youcheng, Z. (May 2003). "Synthesis and analgesic activity of stereoisomers of cis-fluoro-ohmefentanyl". Die Pharmazie. 58 (5): 300–302. PMID 12779044.
- ↑ Brine GA, Carroll FI, Richardson-Leibert TM, Xu H, Rothman RB (August 1997). "Ohmefentanyl and its stereoisomers: Chemistry and pharmacology". Current Medicinal Chemistry. 4 (4): 247–270. ISSN 0929-8673.
- ↑ Mounteney J, Giraudon I, Denissov G, Griffiths P (July 2015). "Fentanyls: Are we missing the signs? Highly potent and on the rise in Europe". International Journal of Drug Policy. 26 (7): 626–631. doi:10.1016/j.drugpo.2015.04.003. PMID 25976511.
External links[edit]
- Ohmefentanyl at the US National Library of Medicine Medical Subject Headings (MeSH)
This article "4''-fluoro-ohmfentanyl" is from Wikipedia. The list of its authors can be seen in its historical and/or the page Edithistory:4''-fluoro-ohmfentanyl. Articles copied from Draft Namespace on Wikipedia could be seen on the Draft Namespace of Wikipedia and not main one.
