Cupex
The CUPEX technology is a copper leaching-electrowinning process that has recently been developed by M.R Bakhshandeh.
The present invention relates, generally, to a method for dissolving copper minerals in a liquid media, especially dilute aqueous sulfuric acid, chloridric acid or ammonia, and then direct electrowinning copper powder from a copper containing solution using anode cathode reactions. The copper metal powder is deposited at the bottom of the cathode while the anodic reaction is a competition between oxygen evolution and ferrous /ferric anode reaction. Afterward, the deposited powders can be classified by size and directly be used or transferred to the smelting house to form anode for electrowinning .[1]
Leaching
The first stage of the CUPEX process is to dissolve the copper content of the raw materials in dilute sulfuric acid, chloridric acid or ammonia which is so-called leaching stage.
There are different types of metallurgical leaching processes according to the physical and chemical properties of the raw ore and the mine characteristics.
- In-situ leaching: dissolving the copper minerals through bore holes drilled into the deposit.
- Dump leaching: dissolving copper minerals from ore taken directly from the mine and stacked on the pad without crushing.
- Heap leaching: dissolving copper from ore which has been crushed into small chunk.
- Tank leaching: the process in which the crushed ore is stocked in some tanks with lead coated walls.
One of the most important parameters in designing the leaching process for copper oxide or is the acid consumption. If acid consumption increases over a critical point, the cost efficiency of the project can be threatened. The average acid consumption is between 10-15 [Kg-H2SO4/tone] copper ore. It is noticeable that the leaching technology of the sulfite ore is quite different from that of oxide ores. Heap leaching is far and away the most important method of hydrometallurgical Cu extraction.
It entails:
- Building flat surface heaps of ore pieces
- Applying aqueous sulfuric solution to the top surface of the heap via an equispaced network of pipes.
- Allowing the solution to trickle through the heap.
- Collecting the Cu2+ rich pregnant solution on a sloped impermeable surface beneath the heap.
- Directing gravity flow of pregnant solution to a pond or tank.
- Sending the collected solution to the electrowinning for metallic powder production.
- Recycling raffinate solution and send it back to the heap for further leaching.
Leach heaps are either multi-lift or on/off.[2]
Electrowinning
The most important characteristic of the CUPEX technology is the exclusion of any solvent extraction or ion exchange stage which in ordinary conventional methods not only cost a great amount but also needs high technology and accurate systems and procedures.
Leaching stage produces CuSO4-H2SO4-H2O electrolyte with 0.5- 2gr/liter Cu2+. Electrowinning of this solution will results in metallic copper powder.
Electrowinning entails:
- Immersing metal cathodes and anodes in CuSO4-H2SO4-H2O electrolyte.
- Applying an electrical potential between the anodes and cathodes.
- Depositing metallic copper powder onto the cathode.
A copper containing solution is introduced to into an electrowinning cell, and copper is electrowon from the solution to form copper powder. A copper powder slurry steam which comprises the copper powder and electrolyte is collected and removed from the electrowinning cell. While a lean electrolyte steam exits the electrowinning cell from a side or top portion of apparatus.
The lean electrolyte exiting the electrowining apparatus maybe subjected to filtration to remove suspended copper particles. Moreover the rich electrolyte entering the electrowinning apparatus may be subjected to filtration prior to electrowinning to remove any undesirable solid impurities.
In order to attain metallic copper powder, low Cu concentration, high acid concentration and high current density need to be provided.
Industrially the anodes are rolled Pb alloy or stainless steel sheets and The cathodes are thin copper sheets. In copper electrowinning process the following reactions occur: Cathode reaction:
2Cu2++4e-→2Cuo Eo =0.34 V
Anode reaction:
2H2O -4e-→4H+ + O2 Eo =-1.229 V
The overall reaction is:
Cu2+ +H2O→2H+ +1/2 O2 +Cuo Eo =-0.889 V
The theoretical potential needed for electrowinning is about 2 volts, it is made up of:
Theoretical voltage for reaction (about 0.9 v)
Oxygen deposition overvoltage (about 0.5 v)
Copper deposition overvoltage (about 0.05 v)
Electrical resistance at 1000 A/m^2 cathode current density (about 0.7 v)
The energy requirement for electrowinning is a function of Cu concentration in solution and decreases by increasing the Cu content of the solution.
W=-2324+89 log(XCu) w/gram Cu
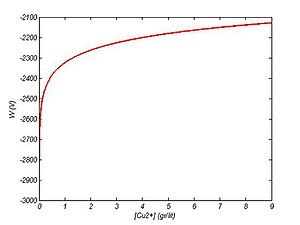
The size of the electrolytic cell, anode and cathode are:
- Cells: polymer concrete
- Anodes: rolled Pb alloy 1.1m long, 0.9m wide, 0.006m thick
- Cathode: copper sheets 1.2m long, 1 wide , 0.003m thick
Cells can be made up of steel walls with polyvinyl chloride or polyethylene linings. Electrolyte flows continuously from an active storage tank into each cell through manifold.
Current density Cathode density should be about 1000-3000 A/m2 this gives a deposition rate of 1.5 kg copper per hour per square meter on each cathode. Copper deposition rate increases with increasing current density.
Electrolyte
CUPEX electrowinning electrolyte typically should contain 0.5-3 gr Cu2+ and 10 gr H2SO4 per liter as it enters the electrolyte cell .
Current efficiency
The current efficiencies in electrowinning plants are about 90% , the current is wasted by:
Anode /cathode short circuits
Stray current to ground
Reduction of Fe3+ to Fe2+ at the cathode and re oxidation of Fe3+ to Fe2+ at the anode.
The current efficiency is important because it affects the energy consumption and copper depositing rate.
Fig 2 shows current efficiency is dependent to copper concentration.
Fig 3 shows a flow sheet of the CUPEX process which starts from the leaching stage and results in copper anode for sale.


References[edit]
This article "Cupex" is from Wikipedia. The list of its authors can be seen in its historical. Articles copied from Draft Namespace on Wikipedia could be seen on the Draft Namespace of Wikipedia and not main one.
