EC5026
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Drug class | Analgesic |
| Pharmacokinetic data | |
| Elimination half-life | 42–59 hrs |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}} |
| Chemical and physical data | |
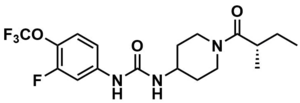
| Formula | C18H23F4N3O3 |
| Molar mass | 405.394 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
EC5026 (BPN-19186) is an inhibitor of soluble epoxide hydrolase (sEH) and a non-opioid investigational analgesic.[1] It entered clinical trials in 2019 as a potential treatment for diabetic neuropathic pain.[2] A Phase 1a trial was completed in 2020 and the drug was well tolerated with no treatment-related adverse events reported.[3] A Phase 1b expansion was opened in 2021.[4]
EC5026 is a reversible, competitive inhibitor of sEH (Ki < 50pM).[1][5]
References[edit]
- ↑ 1.0 1.1 WO 2015/148954, Hammock, B.D., Lee, K.S.S., Inceoglu, B.A, "Potent soluble epoxide hydrolase inhibitors", published 2015-10-01
- ↑ "EicOsis Announces FDA Accepts IND for EC5026 to Treat Pain". PR Newswire. Retrieved 8 November 2021.
- ↑ "NCT04228302". Clinicaltrials.gov. Retrieved 8 November 2021.
- ↑ "NCT04908995". Clinicaltrials.gov. Retrieved 8 November 2021.
- ↑ Hammock BD, McReynolds CB, Wagner K, Buckpitt A, Cortes-Puch I, Croston G; et al. (2021). "Movement to the Clinic of Soluble Epoxide Hydrolase Inhibitor EC5026 as an Analgesic for Neuropathic Pain and for Use as a Nonaddictive Opioid Alternative". J Med Chem. 64 (4): 1856–1872. doi:10.1021/acs.jmedchem.0c01886. PMC 7917437 Check
|pmc=value (help). PMID 33550801 Check|pmid=value (help).CS1 maint: Multiple names: authors list (link)
| This pharmacology-related article is a stub. You can help EverybodyWiki by expanding it. |
This article "EC5026" is from Wikipedia. The list of its authors can be seen in its historical and/or the page Edithistory:EC5026. Articles copied from Draft Namespace on Wikipedia could be seen on the Draft Namespace of Wikipedia and not main one.
